valence electrons of argon|list of valence electrons for each element : Cebu The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration. Knowing the electron configuration in the right way, it is very . Tingnan ang higit pa EEST (Eastern European Summer Time) is 7 hours ahead of Eastern Daylight Time 6:00 pm 18:00 in Cairo, Egypt is 11:00 am 11:00 in EDT. Cairo to EST call time Best time for a conference call or a meeting is between 4pm-6pm in Cairo which corresponds to 8am-10am in EST. 6:00 pm 18:00 EEST (Eastern European Summer Time) (Cairo, Egypt). Offset .
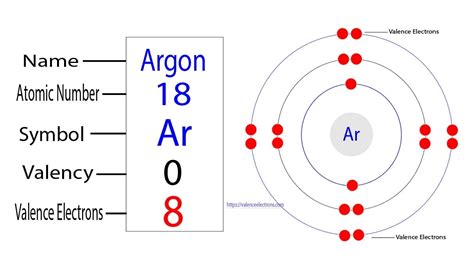
valence electrons of argon,Learn how to calculate the valence electrons of argon (Ar) by following three steps: finding the atomic number, electron configuration, and valence shell. Also, find out the valency, reasons for inertness, and video of argon. Tingnan ang higit paArgon is an element of group-18. The valence electron is the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of argonis called the valence electrons of argon. The valence . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of . Tingnan ang higit paThe valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration. Knowing the electron configuration in the right way, it is very . Tingnan ang higit paThe electron configuration of argon shows that the number of electrons in the last orbit(last shell) of the argon(Ar) atom is 8. We know . Tingnan ang higit pa Mar 23, 2023
There are two ways to find the number of valence electrons in Argon (Ar). The first is to use the Periodic Table to figure out how many electrons Argon has in its valence shell. To do so,. Learn how to identify valence electrons, the electrons in the outermost shell of an atom, and how they affect chemical reactions. See examples of oxygen, argon, and other elements, . The total number of electrons present in the valence shell of an atom is called valence electrons, and there are eight electrons present in the valence shell of argon (3s²3p⁶). Thus, argon has eight . The single dot pair represents the single bond. Double pair of dots represents the double bond. The technique is useful in the proper analysis of electron .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel .
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .

Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital.Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..list of valence electrons for each element There are two ways to find the number of valence electrons in Argon (Ar). The first is to use the Periodic Table to figure out how many electrons Argon has i. 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..

sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency (or valence) of an atom. . Valency of Argon: 18: 0: Valency of Potassium (K) 19: 1: Valency of Calcium: 20: 2: Valency of Scandium: 21: 3: Valency of Titanium: 22: 4: Valency of Vanadium: 23: 5,4 . Explanation: Argon is a noble gas with an electron configuration of [N e]3s23p6. So, it has 3 electron shells, and that will be its valence shell. In the third shell, it holds a total of 2 + 6 = 8 electrons, so it has 8 valence electrons. Answer link. 8 Argon is a noble gas with an electron configuration of [Ne]3s^2\3p^6.valence electrons of argon list of valence electrons for each element Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .This is why third-row elements, such as argon, can be stable with just eight valence electrons: their s and p subshells are filled, even though the entire 3n shell is not. While electron shells and orbitals are closely related, orbitals provide a more accurate picture of the electron configuration of an atom.valence electrons of argonHow to determine the number of valence electrons and draw Lewis structures for main group elements starting from the electron configuration. Created by Jay. . then chlorine would have an electron configuration like a noble gas, like that of argon. So chlorine will gain an electron here. So let's go ahead and write the new electron .
For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic .
Now, the electron configuration of argon shows that the last orbit has eight electrons. Therefore, the valence electrons of argon are eight. The last shell of argon has no unpaired electron, so the . The shorthand electron configuration for Argon is [Ne] 3s 2 3p 6. The number of valence electrons available for the Argon atom is 8. Argon is situated in Group 18th or 8A and has an atomic number of .Argon provides an inert atmosphere in which welded metals will not oxidise. Appearance. Argon is a colourless, odourless gas that is totally inert to other substances. Uses. Argon is often used when an inert atmosphere is needed. It is used in this way for the production of titanium and other reactive elements.The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the .In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms. A fourth subshell, . so neutral phosphorus atoms have 5 valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons.Microsoft Teams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. Hint: Electrons which are present in the outermost shell of the atom are known as valence electrons and for the calculation of the number of valence shell electrons we should know about the electronic configuration of the atom. Complete Solution : Symbolically Argon is denoted as ‘${\text{Ar}}$’ in the periodic table and its .
valence electrons of argon|list of valence electrons for each element
PH0 · valence electrons for kids
PH1 · valence electrons chart
PH2 · valence electrons calculator
PH3 · orbital diagram for argon
PH4 · number of valence electrons list
PH5 · list of valence electrons for each element
PH6 · how do you find valence electrons
PH7 · argon core electrons
PH8 · Iba pa